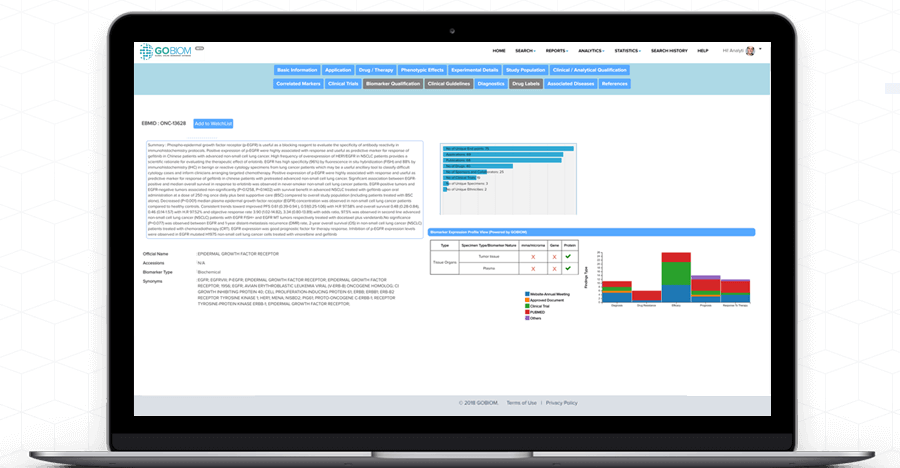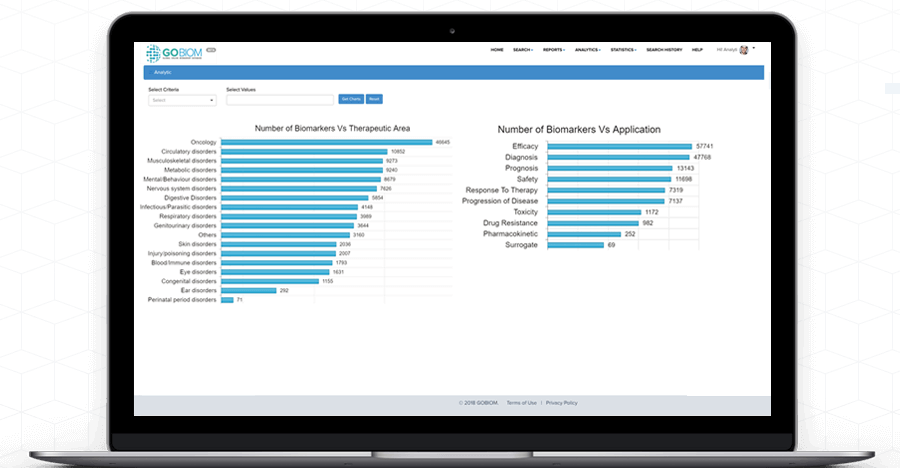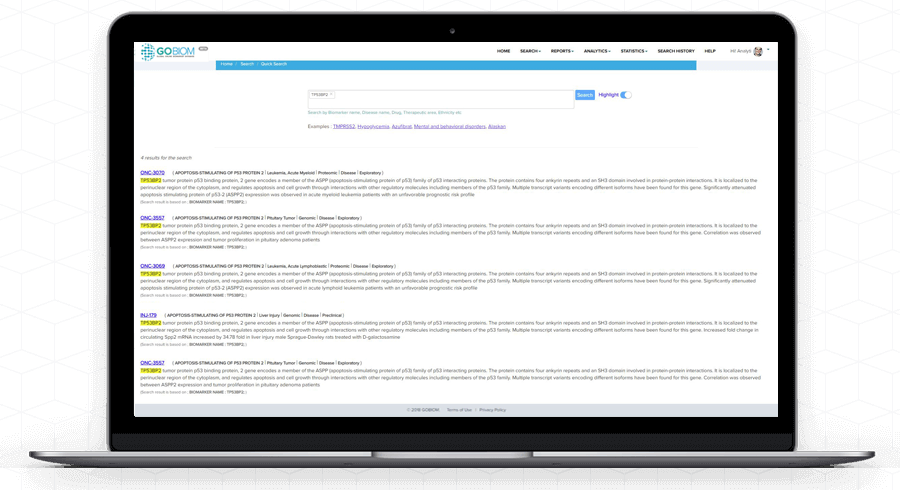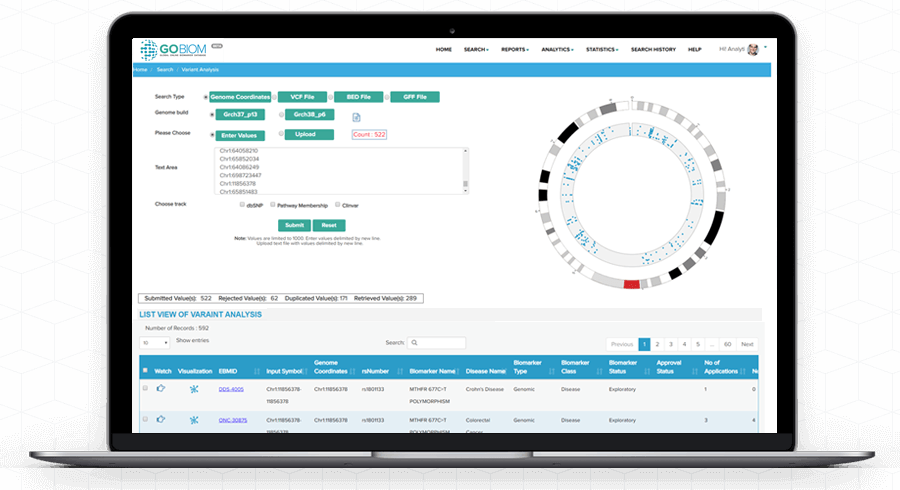


Understand complex 'biomarker data' with GOBIOM
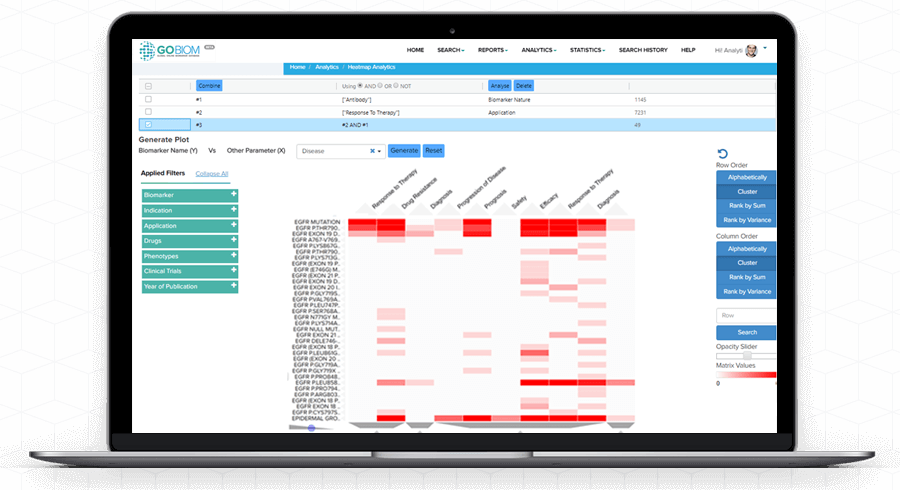
Heatmap
Visualization
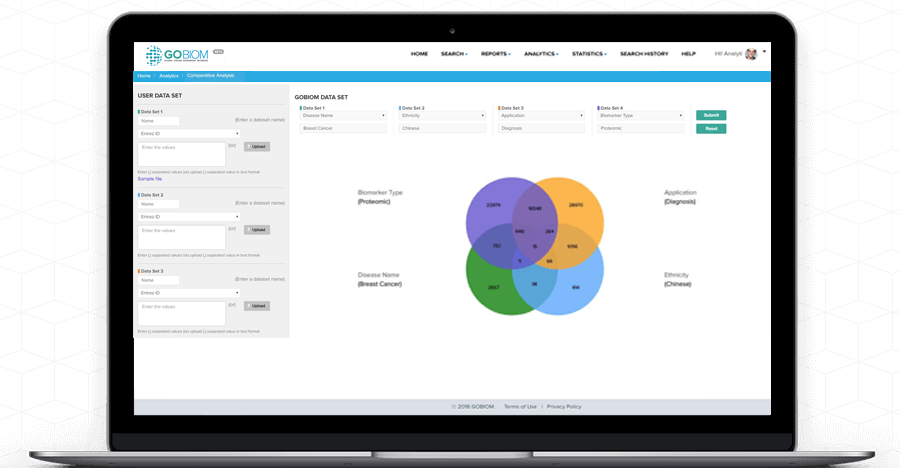
Comparative
Analysis
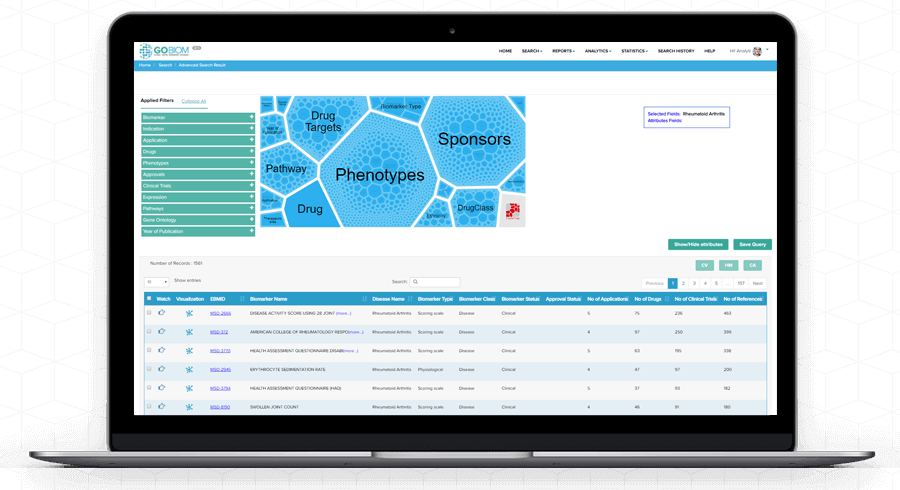
Search
Results
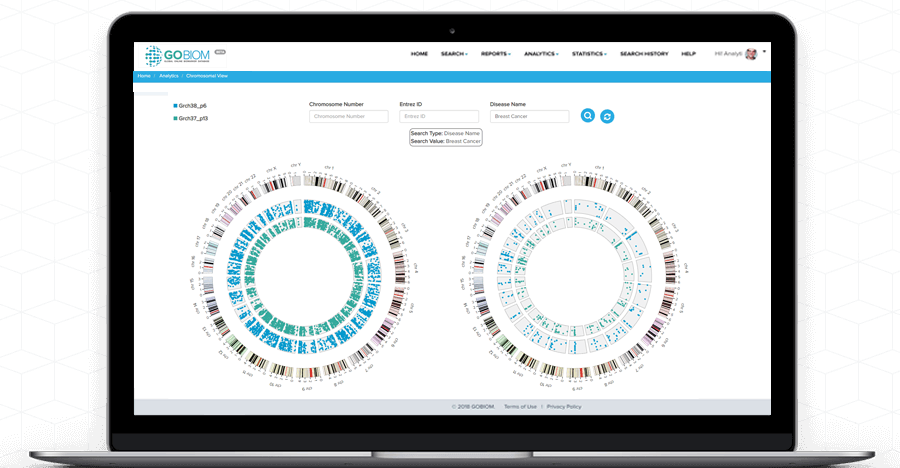
Chromosomal
view
Intuitive
Reports
Dynamic
Dashboards
Quick
Search
Variant
Analysis
GOBIOM Highlights
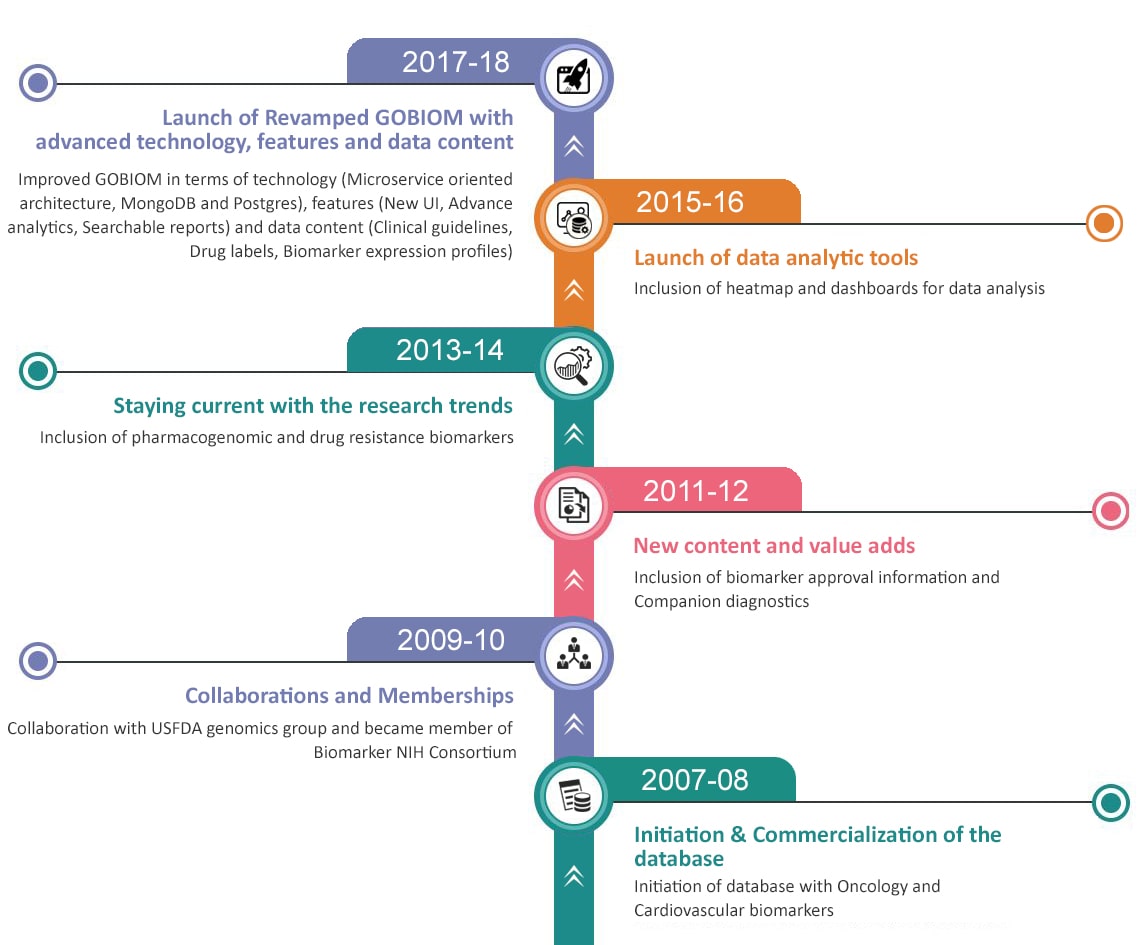
What's New on GOBIOM
Are there any diagnostic biomarkers?
Are there any biomarkers to identify disease progression and prognosis?
What are the markers linked to efficacy/toxicity of a drug?
Are there any biomarkers approved by regulatory authorities?
Are there any evidence based clinical practice guidelines issued by professional societies?
Is the biomarker translating from preclinical to clinical models?
What are the clinical endpoints to which a biomarker is significantly correlated?
What is the study population who would be most benefitted by a therapy?
How many clinical trials are associated with a given biomarker? What is the biomarker outcome in each trial?
What are the approved diagnostics and companion diagnostics for a given biomarker and who are the assay vendors?
What are the drug resistance biomarkers reported for a given therapy?
Are there any off-label uses of the approved drugs?
BIOMARKER UTILITIES
Each biomarker is assigned with the following utilities as reported by the investigatorBiomarkers which indicate the presence or likelihood of a particular disease in
patients
or in animal models.
Ex: Significantly elevated serum carbonic anhydrase 1 (CA1) level is reported
as a novel
early diagnostic biomarker in stage I non-small cell lung cancer (NSCLC) patients
compared
to healthy control subjects
Biomarkers which can discriminate between patients at high and low risk of a
clinical outcome.
Ex: Estrogen receptor, beta in Breast cancer
High epithelial Lysosomal-associated membrane protein 3 (LAMP3) expression is
reported
as a novel biomarker of poor prognosis in patients with esophageal squamous cell
carcinoma
Biomarkers which can indicate the progression of disease.
Ex: Dysregulation of glutathione S-transferase pi 1 (GSTP1) is reported as
novel biomarker
predicting aggressive disease and disease progression in African American men with
prostate
cancer
Biomarkers which correlate with the desired effect of a treatment.
Ex: FEV1 is reported as a efficacy biomarker as evident by its significant
improvement
following treatment with MK-7123 in chronic obstructive pulmonary disease patients
Biomarkers predictive of response to a treatment.
Ex: Whole blood alpha defensin DEFA1 mRNA expression is reported as a
predictive biomarker
of drug response in castration-resistant prostate cancer (CRPC) patients receiving
docetaxel
treatment
Markers which substitute a desired clinical outcome and which can be measured
months or
even years before meaningful clinical endpoints like mortality or morbidity.
Ex: Increased BNP is correlated with the extent of myocardial ischemia, age,
renal insufficiency,
and ventricular dysfunction in patients with acute coronary syndrome suggesting
that
BNP may be useful as a surrogate marker for multiple conventional risk factors.
Indicate potentially harmful effects of a drug in clinical population
Ex: Blood alkaline phosphatase is reported as a safety biomarker as evidenced
by its
increased level in 1.27% gastric cancer patients treated with ramucirumab as
compared
to 0% patients treated with placebo
Indicate potentially harmful effects of a drug in cell-based or preclinical
studies.
Ex: Gentamicin sulfate induced nephrotoxicity was measured in terms of
significantly
increased renal, urinary and serum clusterin levels respectively in adult Lewis
rats
after 12 days of treatment at 100 mg/kg/day
Biomarkers which can be used to indicate the pharmacokinetic properties of the
drug
Ex: Ferritin is reported as a pharmacokinetic biomarker as evident by
significant association
between serum ferritin level and volume of drug distribution of non-conjugated
deferiprone
and urinary iron excretion in splenectomized and non-splenectomized
Beta-Thalassaemia/haemoglobin
E patients treated with a single oral dose of deferiprone at a dose of 25 mg/kg